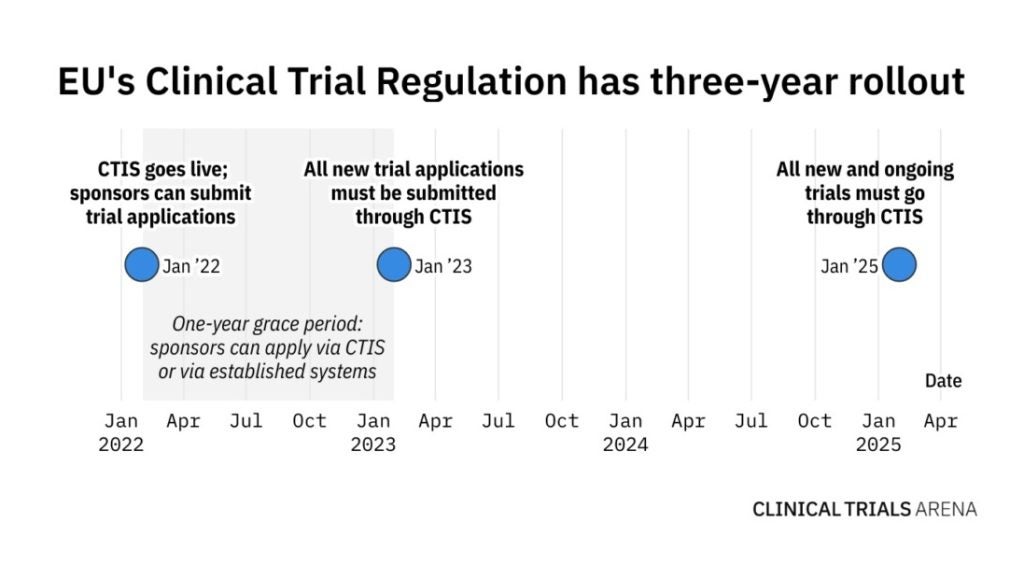
It has been just over four months since EudraCT closed its doors for all new clinical trial applications (CTAs). Following a one-year grace period, sponsors now need to submit CTAs via the not-so-new Clinical Trials Information System (CTIS) and comply with EU Clinical Trials Regulation (EU CTR) that went live in January 2022.
Even though the launch of CTIS and EU CTR was announced several years ago, many are still struggling to adapt to it. Both CTIS and EU CTR are envisioned to streamline the clinical trial application process, as sponsors can submit all regulatory and ethics assessments in one application instead of applying to each EU member state individually.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
When CTIS was launched, many sponsors were hesitant to jump on board immediately. Experts told Clinical Trials Arena in July 2022 that some companies may want to see others try it first before they dip their toes into the portal. Later in the year, the situation improved but still, many sponsors held back in hopes of finishing their studies before the 2025 deadline, and consequently not have to switch.
CTIS uptake so far
According to the EMA’s latest key performance indicator (KPI) report, which was published on 16 May, the number of CTAs submitted in CTIS has jumped since January this year.
Additionally, more sponsors are submitting substantial modifications to their CTAs or subsequently adding a new EU member state.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataWhile there is increased activity from trial sponsors, the EMA and the member states are also getting more proactive with giving feedback. Again, since January there has been an increase in decisions being made, meaning a trial was authorised with or without conditions, or not authorised.
Werner Engelbrecht, senior director of Veeva Vault Clinical Operations strategy, says that the EMA has established a good team to back CTIS and a better structure to support not just technical but also general questions.
But while there has always been a drive to discuss improvements on the EMA level, lately there have also been a lot of changes on the member state level. In order to meet the growing needs, Engelbrecht says that each member state had to set up new ways of working with ethics committees, which was new to them.
Technical limitations of the portal
While the number of instances where new member states are added to CTAs has increased, Engelbrecht says sponsors need to wait for 30 days after the initial submission to add new countries to the application. This means companies need to start changing the way they plan clinical trials and think ahead of time. “It is a strategic decision because it could jeopardise compound development timelines,” he says, adding that this is a technical limitation and not a regulatory one.
Also, CTIS is a portal and does not have any application programming interface (API) capabilities, meaning that a person has to manually input the data, which may lead to human error. Engelbrecht says that the EMA has some API capabilities, but they are only limited to individual member states. Even then, it is not a bidirectional communication system.
API functionality is something the industry would welcome, but the EMA would first need to have a well-established software development environment before opening it up to external users like biotech and pharma companies, and CROs. Based on talks with key people at the EMA, Engelbrecht says regulators are looking into it, but this may not happen in the next few years.
Breaking internal silos
Another pain point for sponsors when it comes to CTIS submissions is the organisational setup for submissions. Sponsors need to clearly identify roles and responsibilities and visualise the end-to-end process, Stephan Ohnmacht, vice president of Veeva R&D Business Consulting, said during a media roundtable at the Veeva R&D Summit held 6–7June in Madrid.
This new operational approach is changing the paradigm, and reshuffling the way organisations operate, which is not easy to implement. Despite the EU CTR being live for several months, Ohnmacht noted some companies still do not want to accept this fact.
Breaking internal silos is especially difficult for large pharma companies as they have traditionally employed different teams or individuals doing one part of the process. “Individual areas were isolated for a good reason, to get things done. But there are some opportunities to improve efficiencies and gain connectivity within the company,” Ohnmacht added.
Indeed, this is the experience that Novo Nordisk shared in a session called “Implementing Process and Technology Changes to Comply with EU CTR” at the Veeva Summit. Majbritt Romme, senior regulatory professional and process expert at the company, said that it became clear early on that to comply with EU CTR, Novo Nordisk had to identify people who would be responsible for different functions like handling documents, uploading them to CTIS, and monitoring CTIS. As such, the company established a new EU submission team.
Engelbrecht explains that big enterprises like Novo Nordisk have a long-standing history of siloed organisational structure. While there was not always a need to have regulatory and clinical environments in full alignment, this is the first time where there is an urgent need to collaborate. He advises bigger companies to seek out external business consulting groups to help identify new ways of working with EU CTR and CTIS.
However, small to medium companies may not need to outsource for advice on EU CTR. Engelbrecht says that such sponsors are more likely to delegate these tasks to CROs or build new operational models with them instead.
Academic sponsors lagging behind
CTIS and EU CTR were released as part of the key priorities outlined in Accelerating Clinical Trials in the EU (ACT EU), an initiative to transform the initiation, design, and conduct of clinical trials in the EU. It was launched together with CTIS and EU CTR in January last year by the European Commission (EC), the Heads of Medicines Agencies (HMA), and the EMA.
One of the ACT EU priorities focuses on large and multinational clinical trials, especially academia-led studies. However, EMA data shows that the commercial sector is still leading in the number of multinational trial applications. Since the launch of CTIS, 209 multinational trial CTAs were submitted by commercial sponsors. There are only 21 multinational CTAs with a decision submitted by academic centres.
Engelbrecht explains that academic institutions, hospitals, and university hospitals struggle with the complexity that EU CTR and CTIS bring to the submission process. Now, there are ethics committees, regulatory submissions, and transparency reporting, and all of the paperwork has to be submitted all at once, which is something they are not used to doing.
To improve this, member states should be accountable in helping academic institutions to get on board with the new directive. Engelbrecht adds that some of the accountability should also be placed on pharma associations to help academic institutions.
Getting ready for 2025
This January marked a historic day of EudraCT closing its doors for all new CTAs, but the old clinical trial registry is still open for ongoing clinical trials. However, after January 2025, all new and ongoing studies will have to be managed on CTIS.
This poses a lot of challenges for sponsors who have trials approved under the old EU Clinical Trial Directive (CTD), meaning that all studies that are not ending by 2025 have to transition to EU CTR. Engelbrecht says that this is an urgent and complex situation, which will require a dedicated workforce to transition studies into CTIS.
While the clinical trials industry is changing amidst different challenges with EU CTR, it is important to remember the original intent of the EMA. The agency wanted to not only harmonise the process, but also increase transparency and give patients access to clinical trial information.
In May, the EMA initiated a public consultation to evaluate transparency rules for publishing clinical trial data submitted via CTIS across the EU. The agency asked stakeholders to share their views by 28 June 2023.
Engelbrecht says that if the information of new and upcoming trials was made more accessible and consumable to patients, it would address a lot of industry’s needs. It comes down to the patient and getting them access as early as possible to this information, he highlights.





