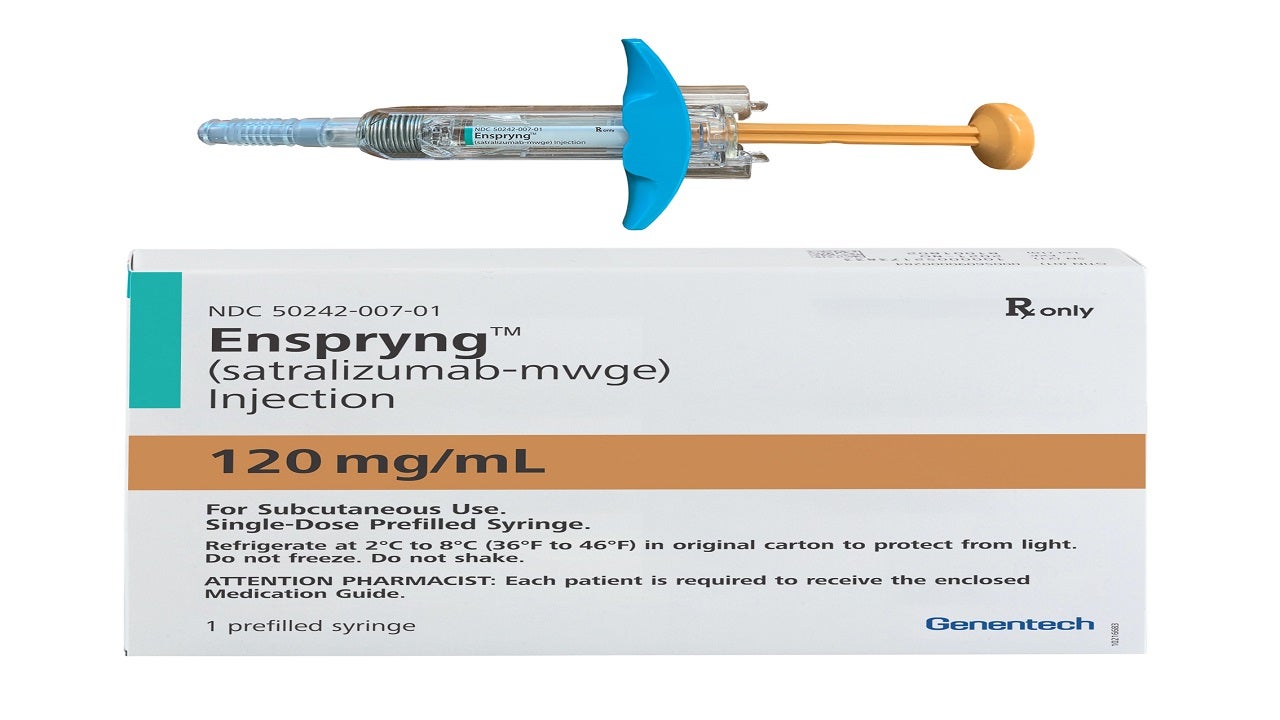
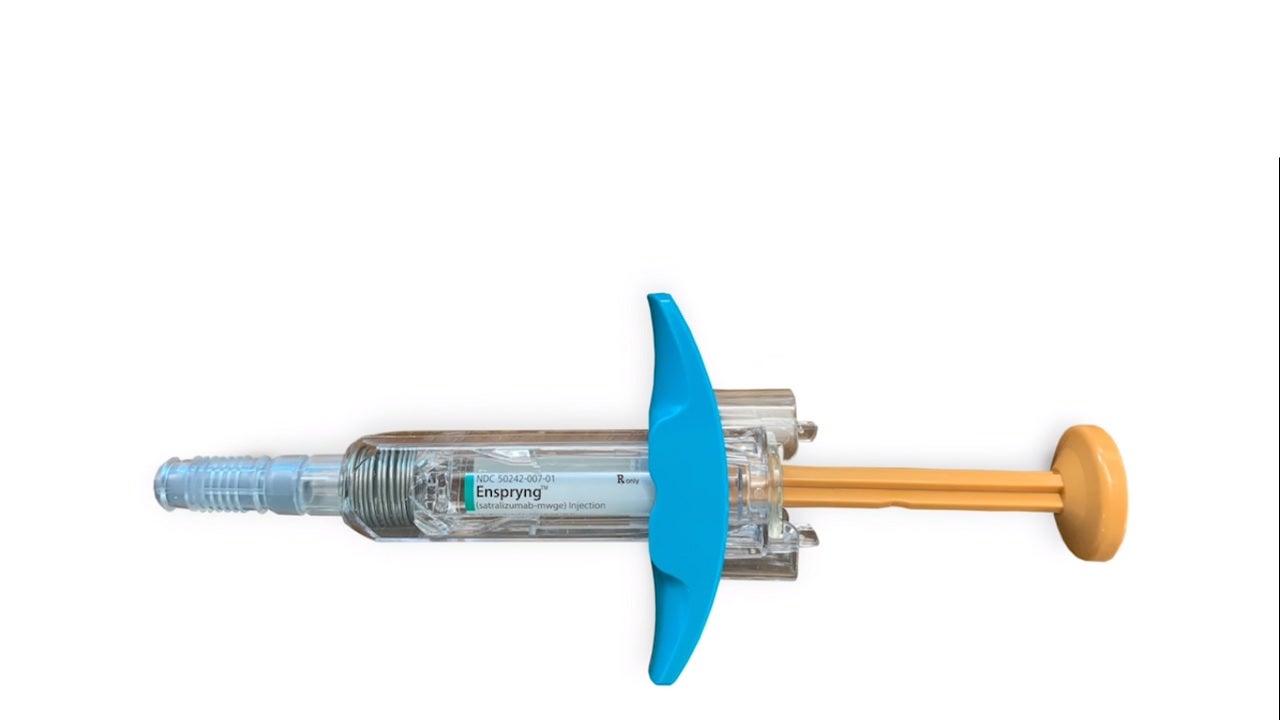
Enspryng™ (satralizumab-mwge) is the first and only US Food and Drug Administration (FDA)-approved subcutaneous therapy indicated for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in anti-aquaporin-4 (AQP4) antibody-positive adult patients.
Developed by Genentech, a subsidiary of Roche, Enspryng is the first and the only NMOSD drug designed to target and block the activity of interleukin-6 (IL-6) receptor, utilising Chugai Pharmaceutical’s new recycling antibody technology.
It is the third approved treatment for NMOSD, after Soliris (eculizumab) and Uplizna (inebilizumab-cdon).
Enspryng is available as a clear, colourless to slightly yellow solution in 120mg / ml dosage strength in a single-dose prefilled syringe for subcutaneous administration.
Enspryng approvals
The biologics license application (BLA) for satralizumab was submitted to the FDA in August 2019, accepted for review in October 2019.
The European Medicines Agency (EMA) accepted the company’s marketing application for the drug for review under Accelerated Assessment in October 2019. China’s Centre for Drug Evaluation (CDE) accepted the licensing application for the drug in April 2020.
Japan’s Ministry of Health, Labour, and Welfare (MHLW) approved Enspryng (satralizumab-mwge) for the treatment of NMOSD in both children and adults in June 2020, while the FDA approved the drug for the treatment of NMOSD in adult patients in August 2020. Enspryng also holds approval in Canada, Japan and Switzerland.
The drug holds FDA’s breakthrough therapy designation, as well as MHLW’s and EMA’s orphan drug designation for neuromyelitis optica (NMO) and NMOSD.
Neuromyelitis optica spectrum disorder (NMOSD) causes and symptoms
NMOSD is a rare and debilitating central nervous system autoimmune disease that predominantly damages the optic nerve and spinal cord, causing blindness, muscle weakness and paralysis.
People with NMOSD experience unforeseen, serious relapses that cause permanent neurological damage, impairment or even death.
NMOSD is generally associated with AQP4s, the pathogenic antibodies that specifically attack and damage astrocytes cells, resulting in inflammatory lesions of the optic nerve, spinal cord and brain.
AQP4 antibodies can be detected in the blood serum of approximately 70% to 80% of NMOSD patients. Most forms of NMOSD can be confirmed by a diagnostic test.
Individuals living with the disorder are often misdiagnosed with multiple sclerosis due to the related features of the two diseases, including greater incidence in women, identical symptoms and relapses.
Satralizumab mechanism of action
Satralizumab is a recombinant humanised monoclonal antibody that targets the function of the IL-6 receptor.
In NMOSD, cytokine IL-6 is assumed to be a key factor for initiating the process of inflammation that leads to damage and impairment.
The exact mechanism of the therapeutic effect of satralizumab in NMOSD is unclear but is assumed to inhibit the IL-6-mediated signalling by binding to soluble and membrane-bound IL-6 receptors.
Satralizumab was designed using Chugai’s proprietary antibody engineering technologies, which allow antibody circulation for a longer time and subcutaneous administration every four weeks compared to traditional technology.
Clinical trials on Enspryng
FDA approval of Enspryng comes from the outcomes of two phase three randomised, placebo-controlled, multi-centre, double-blind clinical trials, SAkuraStar and the SAkuraSky.
In the SAkuraStar study, 95 adult patients enrolled, of which 64 patients were anti-AQP4 antibody positive. The patients were randomised to 2:1 ratio to receive either satralizumab 120mg (n=41) or placebo (n=23) subcutaneously at week 0, 2, and 4, continued at four-week intervals.
The SAkuraSky study enrolled 76 adult patients, of which 52 were anti-AQP4 positive. Patients were randomised to 1:1 ratio to receive 120mg satralizumab (n=26) or placebo added to baseline treatment (azathioprine, mycophenolate mofetil or corticosteroids) (n=26). The therapy was administered subcutaneously at week 0, 2, and 4. The subsequent treatment was continued at four-week intervals.
The time to first protocol-defined relapse (PDR) was the primary goal of the studies.
In SakuraStar study, 55% reduction was observed in the risk of relapses compared to placebo in the combined patient population of aquaporin-4 antibody (AQP4-IgG) seropositive and seronegative patients.
However, in the AQP4 antibody-positive subgroup, 76.5% of Enspryng-treated patients were relapse-free, compared to 41.1% of patients receiving placebo at 96 weeks.
In SAkuraSky study, 91.1% of Enspryng-treated AQP4 antibody-positive subgroup patients were relapse-free compared to 56.8% of patients receiving placebo at 96 weeks.