Lonsurf (trifluridine and tipiracil) for the Treatment of Refractory Metastatic Colorectal Cancer
Lonsurf is approved for the treatment of patients with advanced metastatic colorectal cancer (mCRC).

Gentris Corporation is a leading global provider of applied clinical pharmacogenomic services. As a pioneer in the field of pharmacogenomics, Gentris advances personalized medicine by assisting pharmaceutical companies and clinical research organizations to effectively integrate pharmacogenomics into their drug development programs to deliver safer, more effective drugs to market more quickly.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
Gentris Corporation is a leading global provider of applied clinical pharmacogenomic services. As a pioneer in the field of pharmacogenomics, Gentris advances personalized medicine by assisting pharmaceutical companies and clinical research organizations to effectively integrate pharmacogenomics into their drug development programs to deliver safer, more effective drugs to market more quickly.
Gentris is transforming global drug development with toxicogenomics and clinical pharmacogenomic solutions that can significantly reduce costs and accelerate drug development.
With better characterization of patient populations, pharmacogenomics can help reduce drug failure rates by enabling drug companies to modify patients’ exposure to drugs based on their drug-response genotype. This can offer the added benefit of making drugs that might otherwise not be approved or withdrawn to become available to patients with compatible pharmacogenomic profiles.
Gentris performs pharmacogenomic analysis to identify drugs that may affect safety and efficacy as well as identify patient populations who may have adverse reactions. Gentris’ expertise includes:
Microarray technology allows for comprehensive and global analysis of genetic targets that contribute to, or are predictive of, adverse drug reactions. Using DNA microarray analysis, information can be obtained on the safety and efficacy of your compound. Gentris is DMET®-certified and can perform these studies to help determine patient outcomes and gene associations in preclinical and early phase studies.
Genome-wide association studies can provide information on polymorphisms that may be involved with response, safety and efficacy. For DNA microarray analysis Gentris offers GeneChip® Human 5.0 and 6.0 arrays as well as cytogenetic copy number arrays.
Gene expression analysis can be very helpful in all phases of your trials. Gentris has extensive experience in running microarray expression studies. Gentris offers the Human GeneChip Gene 1.0, Human GeneChip, Genome U133, and GeneChip Human Exon 1.0 ST. In addition, Gentris offers qRT-PCR for gene expression analysis to confirm and validate microarray expression results.
Gentris provides GLP-grade DNA sequencing, high-throughput genotyping, SNP discovery, cancer gene sequencing, microsatellite / STR analysis, SSCP, epigenetics, and the FDA-recommended panel for the cytochrome P450s. Our genotyping results are FDA submissible and our genotyping reports are customizable for easy interpretation.
Gentris has significant expertise in developing custom genotyping and quantitative RT-PCR assays for use with the ABI 7900HT, managing all aspects of the project from assay design to data analysis. By offering pre-optimized validated panels in oncology, CYP450 isozyme expression, cell membrane transporters, and client customized gene panels, Gentris can help maximize therapeutic efficacy, improve prediction of disease stage, prevent clinical onset of toxicity, and identify quantitative biomarkers, as well as assist in drug repositioning.
Gentris has also licensed UGT1A1 from the Mayo Clinic and test patients in clinical trials that will be placed on Irinotecan / Camptosar® therapy for metastatic colorectal cancer. Using a proprietary method, Gentris tests for UGT1A1 *1B, *6, *28, *36, *37, and *60. In addition, Gentris uses the K-ras mutation detection kit from DxS to detect seven key mutations in the K-ras gene: Gly12(Asp, Ala, Val, Ser, Arg, Cys) and Gly13Asp.
Gentris offers toxicogenomic testing using microarray technology. We test various species including human, rat, mice, arapidosis, bovine, Drosphila, Rhesus Macaque, and Xenopus. Gentris’ GeneChip array offering includes:
Gentris also offers qRT-PCR in several genes of several species to examine gene expression levels more quantitatively and aid in biomarker validation.
Gentris has been involved in biostorage for over a decade; we understand the value of your samples. Gentris’ biostorage facility provides 24/7 tracking and reporting, worldwide sample logistics, regulatory compliance, ambient, refrigerated and frozen storage conditions, 21 CFR 11 compliant LIMS, comprehensive project management, and 24hr retrieval.
Gentris is a fully GLP and CLIA-compliant laboratory. You can be assured that your sample will be handled properly with our rigorous chain of custody procedures, sample inventory database, and our compliant biostorage facility. Quality control steps are incorporated throughout Gentris’ sample preparation, testing and storage processes. Internal quality audits are conducted during all key phases of clinical sample processing.

Lonsurf is approved for the treatment of patients with advanced metastatic colorectal cancer (mCRC).
Bydureon® BCise™ (exenatide extended-release) is an injectable suspension containing a glucagon-like peptide-1 (GLP-1) receptor agonist.
Siklos® is an orally administered tablet form of hydroxyurea that is indicated for the treatment of sickle-cell anaemia in paediatric patients aged two years and older.
Endari™ is an orally administered powdered form of amino acid L-glutamine indicated for the treatment of sickle-cell disease.
LuxturnaTM (voretigene neparvovec) is approved for the treatment of patients with biallelic RPE65 mutation-associated retinal dystrophy.
Aliqopa (copanlisib) is a novel intravenous phosphatidylinositol-3-kinase (PI3K) inhibitor indicated for the treatment of adult patients with relapsed follicular lymphoma (FL).
Radicava™ (edaravone) is a neuroprotective agent indicated for the treatment of amyotrophic lateral sclerosis (ALS).
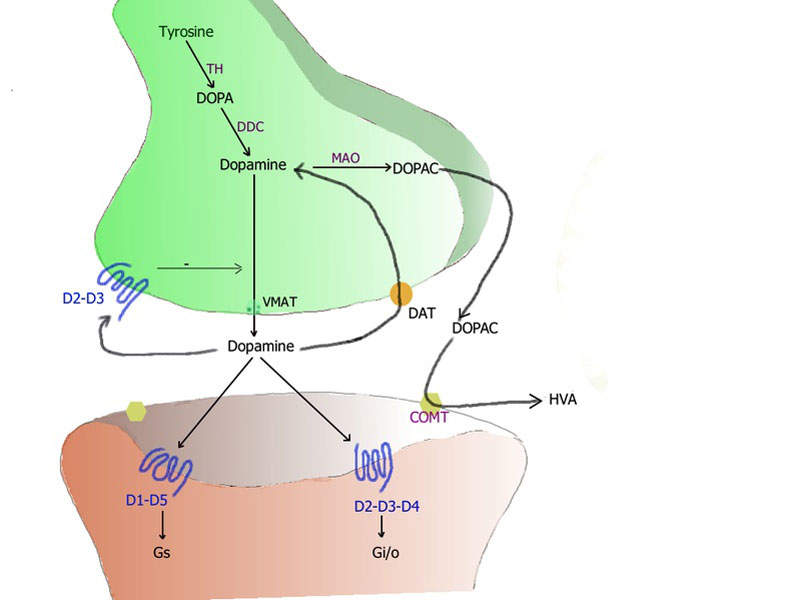
Xadago® (safinamide) is a monoamine oxidase type B (MAO-B) inhibitor indicated for the treatment of Parkinson's disease.