Bydureon® BCise™ for the Treatment of Type-2 Diabetes
Bydureon® BCise™ (exenatide extended-release) is an injectable suspension containing a glucagon-like peptide-1 (GLP-1) receptor agonist.

Exploristics provides innovative analysis solutions to extract information from clinical data. Exploristics has expertise in the exploratory analysis and clinical application of high-dimensional data including biomarkers, pharmacogenomics, imaging and observational data.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
At Exploristics we provide innovative analysis solutions to maximise the information derived from your clinical data. We offer a unique expertise in the exploratory analysis and clinical application of biomarkers, pharmacogenomics, imaging and observational data.
The team at Exploristics offers more than a statistical analysis and reporting service. We take pride in using our analytical skills and scientific expertise to help you make informed decisions. We work closely with our customers to understand their data analysis needs and to provide practical solutions. So far, we have worked successfully with pharmaceutical, biotechnology, medical diagnostics and devices companies as well as healthcare providers. Our service is friendly and cost-effective and our work is compliant with GCP / ICH guidelines.
Our broad experience using multiple analysis techniques enables us to perform a wide range of exploratory data analyses. These allow us to gain a thorough understanding of the data and to detect meaningful patterns in the data.
Furthermore, we can develop mathematical models that describe complex processes and then study the behaviour of those models under different conditions. This approach can save time and money by allowing you to evaluate your options by running virtual experiments. These methods are useful for solving complex problems that involve numerous factors, such as the design of complex studies.
We are experts in the clinical application of biomarkers and pharmacogenomics (PGx). We help you integrate these data into clinical development, evaluate your portfolio to find opportunities for these data, design experiments, analyse data and provide information on how to use the results. We also work with you to enable the integration of these data into your research programs.
We also provide analysis support for the following study types and activities: epidemiology; pre-clinical studies; translational medicine; experimental medicine; medical devices; pharmacovigilance; in-licensing support; and training, education and consultancy.
Exploristics has partnered with OmicSoft Corporation to supply and distribute biomarker data management, visualisation and analysis tools in Europe. OmicSoft has designed software that is easy to use for the bench scientists, but powerful enough to be used by the bioinformatician or statistician. Exploristics offers two software packages: Array Studio and Array Server.
Array Studio provides state-of-the-art statistics and visualisation for the analysis of high dimensional data. Array Studio supports all popular microarray platforms (Affymetrix, Agilent, Illumina, etc.), as well as SNP / genotype platforms (Affymetrix, Illumina, etc.) and CNV platforms (Affymetrix, Illumina and Agilent). Array Studio provides a full workflow for importing and normalisation of data, quality control, analysis, and clustering and pattern analysis.
Array Server is an enterprise solution, allowing users to store, share, search and integrate their microarray / SNP / CNV projects. Array Server supports any number of high dimensional platforms, including the same platforms supported by Array Studio. Users can search by meta data, use search-by-gene functions, and can even profile gene regions across multiple studies.
Bydureon® BCise™ (exenatide extended-release) is an injectable suspension containing a glucagon-like peptide-1 (GLP-1) receptor agonist.
Bavencio® (avelumab) is a human PD-L1 monoclonal antibody indicated for the treatment of metastatic Merkel-cell carcinoma (MCC).
Mydayis is a CNS stimulant drug indicated for the treatment of attention deficit hyperactivity disorder (ADHD) in patients aged 13 years and above.

Rydapt® (midostaurin) is a multi-targeted inhibitor of multiple kinases including FMS-like tyrosine kinase 3 mutation-positive (FLT3+) and KIT approved for the treatment of acute myeloid leukaemia (AML) and three types of advanced systemic mastocytosis (SM) indications.
Ingrezza (valbenazine) is first and only US Food and Drug Administration (FDA) approved vesicular monoamine transporter 2 (VMAT2) inhibitor indicated for the treatment of adults with Tardive dyskinesia (TD).
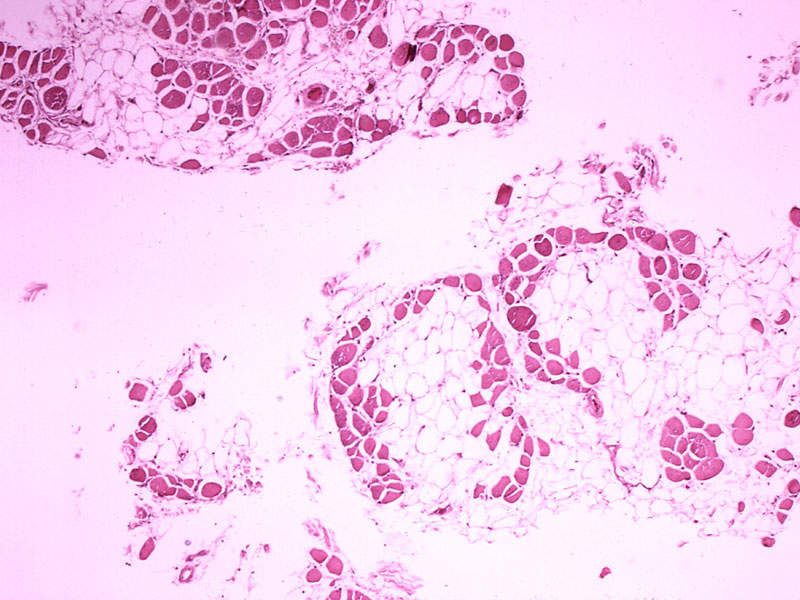
Translarna™ (ataluren) is a protein restoration therapy indicated for the treatment of nonsense mutation Duchenne muscular dystrophy (nmDMD). The drug was developed by PTC Therapeutics.
Formerly known as LEE011, Kisqali® (ribociclib) is a cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor indicated for the treatment of hormone receptor positive, human epidermal growth factor receptor 2 negative (HR+/HER2-) metastatic breast cancer in post-menopausal women.
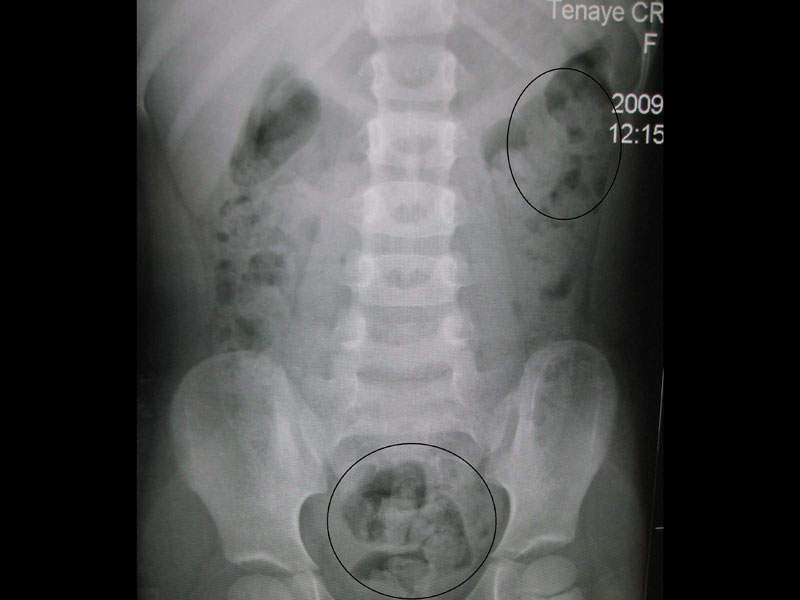
Trulance™ (Plecanatide) is a guanylate cyclase-C (GC-C) agonist indicated for the treatment of adult patients with chronic idiopathic constipation (CIC).

Developed by Novo Nordisk, Xultophy 100/3.6 is indicated for the treatment of type 2 diabetes.
Zinplava (bezlotoxumab) is an injectable solution developed by Merck indicated for the treatment of clostridium difficile infection (CDI) in adult patients who are already receiving anti-bacterial drug treatment.