Bayesian Study Design Offers a Pragmatic Solution for Phase II Clinical Development
Quanticate has released a free-to-download white paper entitled ‘Bayesian Study Design: The Pragmatic Solution for Phase II Clinical Development’.
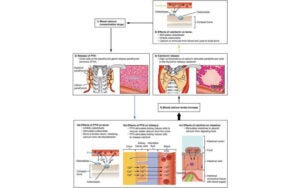
Statistically powered studies in phase II clinical development can be large and expensive, and may not be appropriate at a stage in development where much remains unknown about the likely magnitude of benefit of the compound under study. Pilot studies with small sample sizes may be easier and cheaper to run but are unlikely to yield statistically sound results.
An alternative approach, designing such studies in a Bayesian framework, enables clinical development teams to plan studies that are practical, appropriately sized, and with a clear understanding of the statistical robustness of the results and of the chances of success and failure.
Click here to find out more.