Lucemyra (lofexidine) for the Management of Opioid Withdrawal Symptoms
Lucemyra™ (lofexidine) is a non-opioid medication indicated for the mitigation of opioid withdrawal symptoms.

You have successfully submitted your enquiry. Someone from our company will respond ASAP
Clinical Performance Partners (CPP) is a consulting firm specializing in enrollment and site performance optimization and fostering productive sponsor-site relationships for clinical trials.
We help you evaluate the true operational feasibility of a given protocol and design effective strategies for implementation success. From recruitment and retention planning to effective site training, communications and relationship management programs, we are focused on keeping your study out of rescue mode.
For studies that are behind in enrollment or suffering a range of productivity and compliance symptoms we also help you diagnose, troubleshoot and rejuvenate these trials to get them back on track.
Following a traditional approach to study feasibility assessment, site selection, site training and patient recruitment planning results in traditional results whereby some 20%-30% of the sites enroll 70% of the patients, enrollment falls behind and sites experience study fatigue. Wasting precious time and resources is simply not sustainable in today’s competitive and economically challenged environment.
Simple fixes to these processes yield big results. Through our protocol optimization workshops we help you identify and overcome the implementation hurdles that are likely to impact site performance and patient participation in your trial.
Through our development and facilitation of meaningful investigator meetings and site training programs we help you set the sites up for success with the information and tools they need to effectively implement your trial. With our action-oriented recruitment and retention plans we enable you to design and implement cost-effective recruitment and retention support programs.
While it’s tempting to treat the symptoms of slow enrollment or study fatigue, as the saying goes, ‘prescription without diagnosis is malpractice’. CPP can help you conduct an efficient and effective root-cause analysis of the study performance issues and recommend cost-effective solutions for getting your study back on track. We can help you design and deliver unique and effective rejuvenation meetings and implement compliant site relationship management programs to re-engage your sites.
Simply put, we understand what it takes to get you out of rescue mode.
Lucemyra™ (lofexidine) is a non-opioid medication indicated for the mitigation of opioid withdrawal symptoms.
Ingrezza (valbenazine) is first and only US Food and Drug Administration (FDA) approved vesicular monoamine transporter 2 (VMAT2) inhibitor indicated for the treatment of adults with Tardive dyskinesia (TD).
Xadago® (safinamide) is a monoamine oxidase type B (MAO-B) inhibitor indicated for the treatment of Parkinson's disease.
Anthim (obiltoxaximab) is an injectable drug developed by Elusys Therapeutics for the treatment of inhalational anthrax caused by bacillus anthracis.
Halaven (eribulin mesylate) is an injectable chemotherapy drug developed by Eisai for the treatment of unresectable or metastatic liposarcoma patients that previously received anthracycline-based chemotherapy.
Developed by Amgen, Imlygic (talimogene laherparepvec) is the first injectable formulation of altered herpes simplex virus type 1 for the treatment of cutaneous, subcutaneous and nodal lesions in patients with melanoma recurrence after initial surgery.
Strensiq (asfotase alfa) is the first enzyme replacement therapy (ERT) approved in the US for the treatment of perinatal / infantile and juvenile-onset hypophosphatasia (HPP), which is a life-threatening and ultra-rare metabolic disorder.
Onivyde (MM-398 / irinotecan liposome injection) is an intravenous injection indicated for the treatment of metastatic pancreatic cancer.
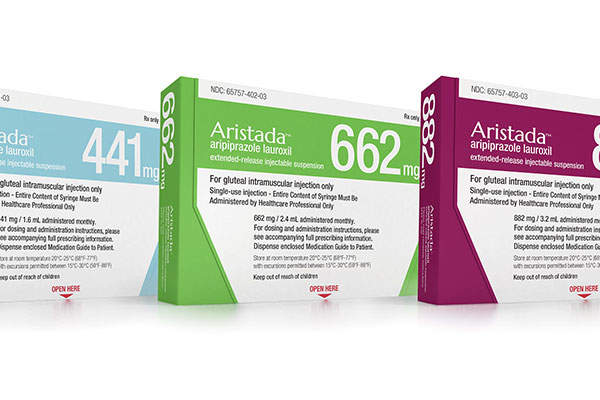
Aristada™ (aripiprazole lauroxil), discovered and developed by Alkermes, is an antipsychotic drug indicated for the treatment of schizophrenia in adults.