Symbicort (budesonide and formoterol) for the Treatment of Asthma
Symbicort is a combination drug comprising an inhaled corticosteroid and a long-acting bronchodilator to treat asthma.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
Onorach Clinical is a clinical trial support company specialising in supporting our clients’ set up and delivery of clinical trials covering Phases I to IV. We’re also experienced in clinical trial services for medical devices and cell-based therapeutics.
We’re a young, fast-growing company focused on providing a fresh approach to our industry. Based in the north-east of Scotland, UK, but operating globally, we offer our clients the same high level of service anywhere in the world.
We pride ourselves on our growing reputation for our pro-active, tailored approach in delivering comprehensive and innovative solutions to the challenges faced in clinical trials.
A strong customer focus and detailed knowledge are at the heart of everything we do; that’s why we’re confident that we can help you deliver your clinical trial efficiently, cost-effectively and to the very highest standards.
Our Right-Sites service is designed to identify, qualify and select the right sites for your clinical trial. This is a complex issue and involves the assessment of a number of factors including:
An evaluation of these and other factors in our Right-Sites service makes for a stronger and more robust project and improves delivery of project time lines within budget.
Finding the optimal principal investigator (PI) is one of the biggest challenges in clinical trials. Not only must they be expert in the required therapeutic area, and have up-to-date experience in Good Clinical Practice (GCP), but they must also be enthusiastic about your trial and prepared to discuss issues with the CRO / sponsor as they arise to ensure the trial remains on time and on budget.
Even the best and most sought-after PIs only have so many hours in a day so we make sure that our PIs are ready to start the project when you are. Our Optimal» PI service addresses all of these issues to ensure the optimal progress of your clinical trial.
At Onorach we look beyond traditional sponsor / CRO models to provide the solutions that sponsors want for clinical trial challenges.
Onorach’s clinical and medical liaison service (OCMLS) is one such solution and is designed to maintain the balance between clinical objectives and business, with Onorach providing the communication bridge between research consultants, sponsor staff and all members of the research team. This takes the communications burden away from the drug development company and ensures that timely communications on-site will prevent minor issues escalating into larger problems which will affect a sponsor and study time-lines.
At a time when the industry is undergoing dynamic changes OCMLS is one of the most effective means of shortening the time to peak study optimisation.
All of the above services can be taken separately or in combination, and we also provide a full range of traditional CRO services such as monitoring and project management.
Our aim is to partner with sponsors in the drug development process, helping our clients address any issue related to the clinical trial process. For a relationship so close, it is important to choose the right partner. Our CRO-sponsorship relationship is one of a shared vision, complementary expertise and trust.
We firmly believe our people are our product; that’s why we invest in equipping our team with the most comprehensive training and skill sets available. Every member of our team is ICH-GCP compliant and works to the highest ethical and professional standards, and our team have experience in a wide range of therapeutic areas.
Call or email us now to find out how we can help you.

Symbicort is a combination drug comprising an inhaled corticosteroid and a long-acting bronchodilator to treat asthma.

Eskata® (hydrogen peroxide) is a topical, non-invasive treatment for raised seborrheic keratoses (SK) in adults.
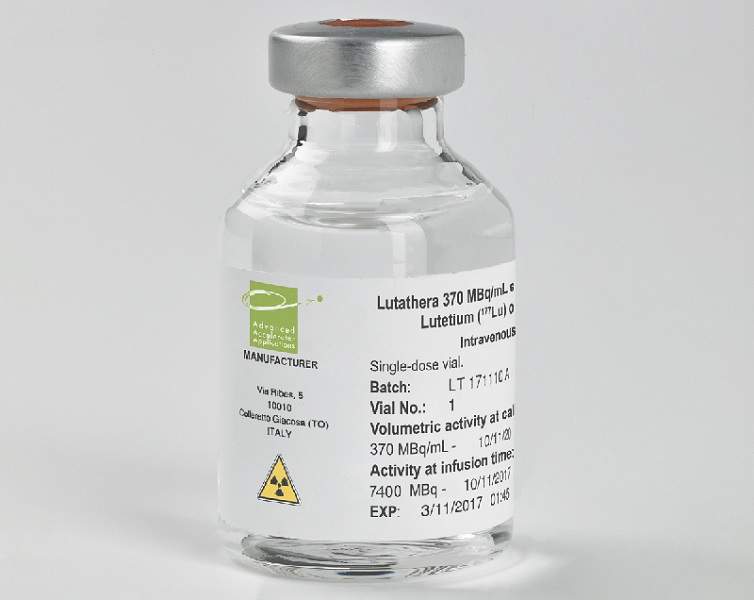
Lutathera® (lutetium Lu 177 dotatate) is a peptide receptor radionuclide therapy (PRRT) indicated for the treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumours.
Faslodex (fulvestrant) is an oestrogen receptor antagonist indicated for the treatment of locally advanced or metastatic breast cancer.
LuxturnaTM (voretigene neparvovec) is approved for the treatment of patients with biallelic RPE65 mutation-associated retinal dystrophy.
Austedo™ (deutetrabenazine) is a vesicular monoamine transporter 2 (VMAT 2) inhibitor indicated for the treatment of chorea associated with Huntington’s disease.
KamRAB/KedRAB™ is a human rabies immunoglobulin (HRIG) indicated for the treatment of passive, transient post-exposure prophylaxis (PEP) of rabies infection. The drug was jointly developed by Kamada and Kedrion Biopharma.
Baxdela™ (delafloxacin) is a fluoroquinolone antibiotic drug indicated for the treatment of adults with acute bacterial skin and skin structure infections (ABSSSI). The drug was discovered and developed by Melinta Therapeutics in partnership with Ligand Pharmaceuticals.
Mydayis is a CNS stimulant drug indicated for the treatment of attention deficit hyperactivity disorder (ADHD) in patients aged 13 years and above.
Tecentriq® (atezolizumab) is a monoclonal antibody designed to bind with a protein called PD-L1.