Symbicort (budesonide and formoterol) for the Treatment of Asthma
Symbicort is a combination drug comprising an inhaled corticosteroid and a long-acting bronchodilator to treat asthma.

You have successfully submitted your enquiry. Someone from our company will respond ASAP
Pharmalys is a UK-based contract research organisation (CRO) that offers a range of clinical trials services. With more than 45 years of combined experience in clinical operations within the pharmaceutical industry, we have extensive expertise across a wide range of therapeutic areas. Through a network of strong partners, we provide innovative solutions throughout Europe and can cover all clinical trial phases in a proactive and cost-conscious way.
We can manage local and international projects from the trial feasibility assessment right through to study reporting. Alternatively, we can offer stand-alone services, giving our customers just the level of assistance they require to complement their in-house competencies.
As part of our clinical trials project management service, we provide you with a dedicated project manager, who is your primary point of contact throughout the entire clinical trial. He/she works hand in hand with your project team to develop an individualised approach to your specific project.
The preparation of applications to ethics committees (EC) and competent authorities (CA) is a specialised regulatory task. Our staff is experienced in managing the approval processes. Our regulatory submission services include:
Our clinical research associates (CRAs) act as the main line of communication between investigators and your departments. While always ensuring subjects’ wellbeing, rights and data reliability, they uphold the image and values of your company at all times. All clinical monitoring activities are conducted according to the international standards of ICH/GCP.
We perform quality assurance audits on all processes used in the management of clinical trials to assess compliance with SOPs, GCP, regulatory requirements, trial protocol, client instructions and contractual obligations. We perform the following types of audit:
We can help design a strategic programme to address recruitment barriers and challenges specific to a given trial protocol, and/or identify the leverage opportunities in order to accelerate patient recruitment.
Following a detailed review of the trial protocol and assessment of the patient population and their carers, we generate a list of tactics along with the areas in which they can be implemented. Each action and its pros and cons are discussed within the project team, leading to a consolidated action plan.
Our database of investigators allows us to conduct a realistic feasibility assessment at very short notice, leading to the selection of the best investigational sites for clinical trials, the correct number of sites and accurate patient recruitment rates.
We understand the importance of selecting appropriate, qualified and productive investigational sites, which is why we put a particular emphasis on site identification. We can take full responsibility for the initial selection of investigational sites or combine your own database of potential investigators with our lists.
Pharmalys has forged partnerships with well-established biometrics companies in order to provide a full range of biometrics services. We can deliver a customised and regulatory-compliant solution to ensure a successful completion of your trial. Our biometrics services include:
Our medical writers have substantial regulatory and interdisciplinary experience to support your projects. We can prepare protocols, investigator brochures, paediatric investigational plans, patient information sheets and informed consent forms, presentations for scientific advice meetings with local and international regulatory bodies, and trial reports.
We can provide strategic or operational clinical trial consultancy services. We offer straightforward, pragmatic advice, enabling you to achieve your project’s ultimate goal using the most efficient route.
Our services include scientific, regulatory, operational and business consulting for programme / trial planning and conduct, process design and improvement, resource planning and management, and vendor selection and management.

Symbicort is a combination drug comprising an inhaled corticosteroid and a long-acting bronchodilator to treat asthma.

Eskata® (hydrogen peroxide) is a topical, non-invasive treatment for raised seborrheic keratoses (SK) in adults.
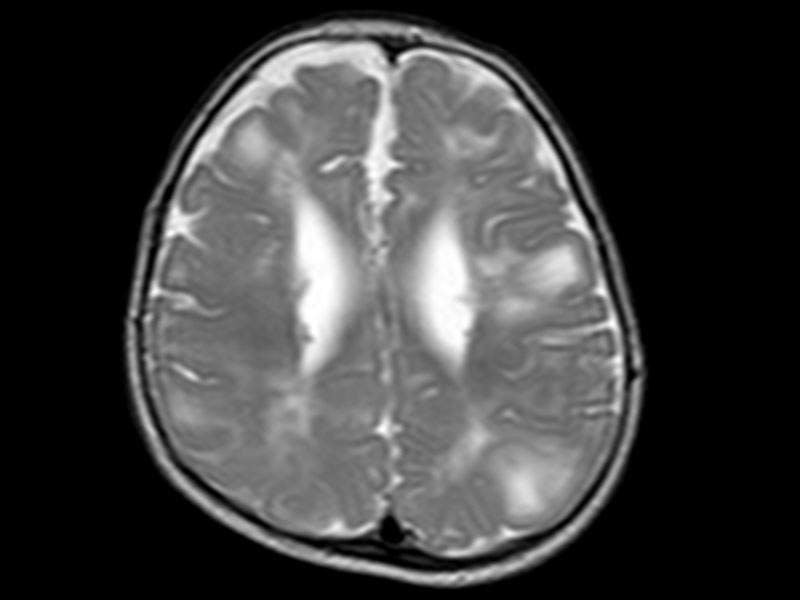
Afinitor Disperz® (everolimus) is indicated for the treatment of tuberous sclerosis complex (TSC) associated partial-onset seizures in adult and paediatric patients.
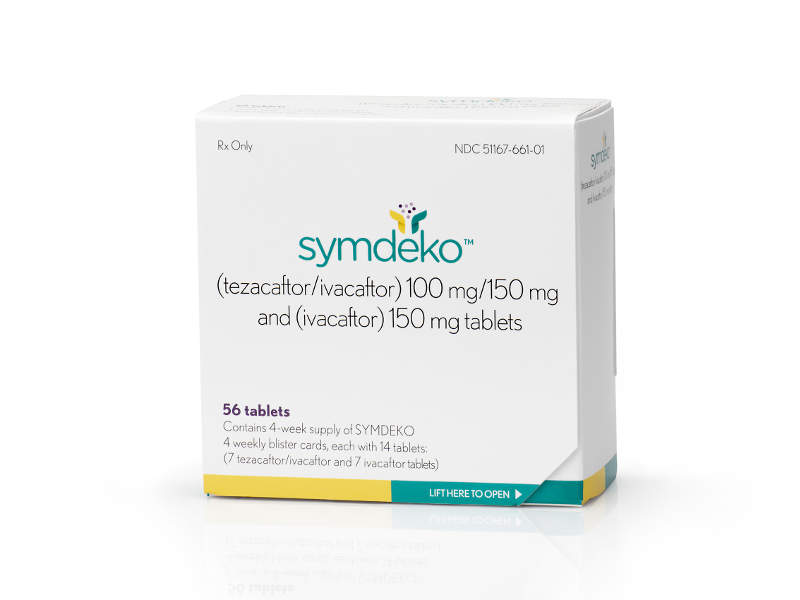
Symdeko™ (tezacaftor/ivacaftor and ivacaftor) is a combination drug indicated for the treatment of cystic fibrosis (CF) in people aged 12 years and above.
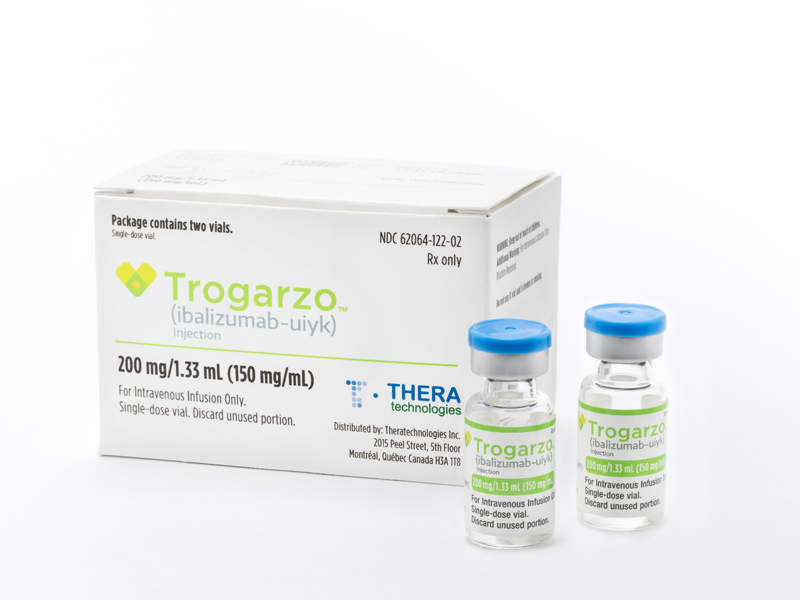
Trogarzo™ is indicated for the treatment of multidrug-resistant HIV-1 infection in heavily treatment-experienced patients.
Admelog® (insulin lispro) is a rapid-acting human insulin analogue approved as a follow-on product to treat type 1 and type 2 diabetes in adults and paediatric patients aged three years and older.
Sublocade™ (buprenorphine extended-release) is a partial agonist indicated for the treatment of moderate-to-severe opioid use disorder (OUD) in adults.
KamRAB/KedRAB™ is a human rabies immunoglobulin (HRIG) indicated for the treatment of passive, transient post-exposure prophylaxis (PEP) of rabies infection. The drug was jointly developed by Kamada and Kedrion Biopharma.

Rydapt® (midostaurin) is a multi-targeted inhibitor of multiple kinases including FMS-like tyrosine kinase 3 mutation-positive (FLT3+) and KIT approved for the treatment of acute myeloid leukaemia (AML) and three types of advanced systemic mastocytosis (SM) indications.

Spritam (levetiracetam) is an adjunctive therapy indicated for the treatment of seizures in patients with epilepsy, the fourth most common neurological disorder affecting people of all age groups.